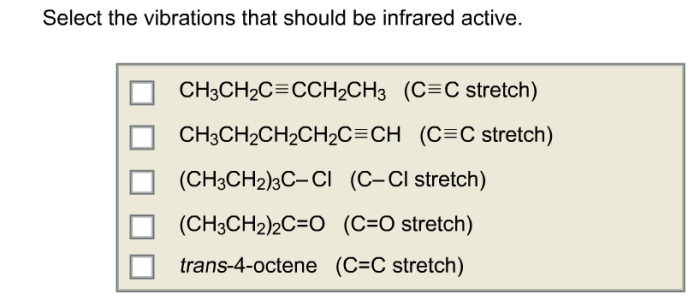
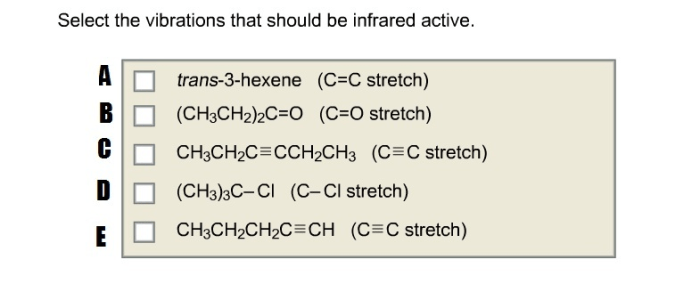
Select the vibrations that should be infrared active. – Infrared spectroscopy, a powerful analytical technique, hinges upon the selection of vibrations that are infrared active. Understanding the principles governing infrared activity is crucial for effectively utilizing this technique. This article delves into the relationship between vibrational modes and infrared activity, exploring the influence of molecular symmetry and functional groups.
We will examine the selection rules for infrared activity based on molecular symmetry and delve into the concept of dipole moment change. Methods for identifying infrared active vibrations in molecules will be discussed, highlighting the use of group theory and character tables.
Finally, we will explore the diverse applications of infrared spectroscopy, demonstrating its utility in identifying and characterizing functional groups.
Vibrational Modes and Infrared Activity
Infrared spectroscopy relies on the absorption of infrared radiation by molecules, causing vibrational transitions. The activity of a vibrational mode in infrared spectroscopy depends on its ability to induce a change in the molecular dipole moment. This change in dipole moment is directly related to the symmetry of the molecule and the functional groups present.
Selection Rules for Infrared Activity
The selection rules for infrared activity are based on the symmetry of the molecule. A vibrational mode is infrared active if it results in a change in the dipole moment of the molecule. This means that the vibrational mode must have a component along the z-axis (the axis perpendicular to the molecular plane).
Additionally, the vibrational mode must be in a symmetry species that allows for a change in dipole moment along the z-axis.
Identifying Infrared Active Vibrations, Select the vibrations that should be infrared active.
There are several methods for identifying infrared active vibrations in molecules. One method is to use group theory. Group theory can be used to determine the symmetry of a molecule and the symmetry species of its vibrational modes. Once the symmetry species of a vibrational mode is known, its infrared activity can be determined using the selection rules.
Applications of Infrared Spectroscopy
Infrared spectroscopy is a powerful tool for identifying and characterizing functional groups in molecules. It is used in a wide variety of fields, including chemistry, biology, and materials science. Infrared spectroscopy can be used to identify the presence of specific functional groups, determine the structure of molecules, and study the dynamics of molecules.
Helpful Answers: Select The Vibrations That Should Be Infrared Active.
What is the relationship between vibrational modes and infrared activity?
Infrared activity is determined by the change in dipole moment associated with a vibrational mode. If the dipole moment changes during a vibration, the mode is infrared active.
How do molecular symmetry and functional groups influence infrared activity?
Molecular symmetry can restrict the number of infrared active vibrations. Functional groups with strong dipoles, such as carbonyl groups, typically give rise to strong infrared bands.
What methods can be used to identify infrared active vibrations?
Group theory and character tables can be used to predict the infrared activity of vibrations in molecules.