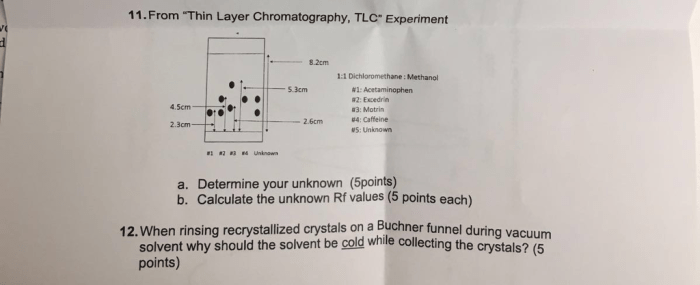
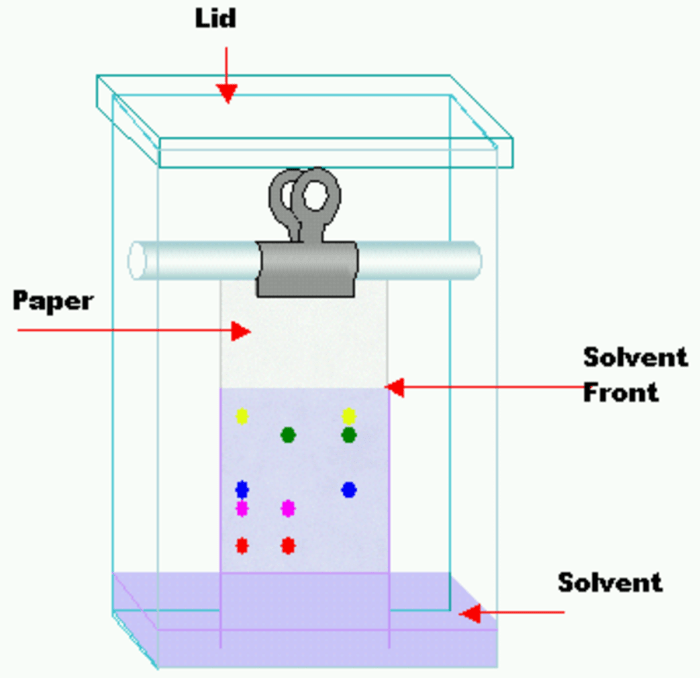
Thin layer chromatography exam questions are a fundamental aspect of assessing students’ understanding of this essential technique. This comprehensive guide delves into the intricacies of TLC, providing a thorough overview of its principles, techniques, applications, optimization, troubleshooting, and data analysis.
By exploring these exam questions, students can solidify their knowledge and enhance their problem-solving skills in the field of chromatography.
TLC, a powerful analytical technique, involves the separation of compounds based on their different interactions with a stationary and mobile phase. Its versatility extends to various scientific disciplines, making it a valuable tool for identifying and quantifying compounds in complex mixtures.
Thin Layer Chromatography (TLC) Principles: Thin Layer Chromatography Exam Questions
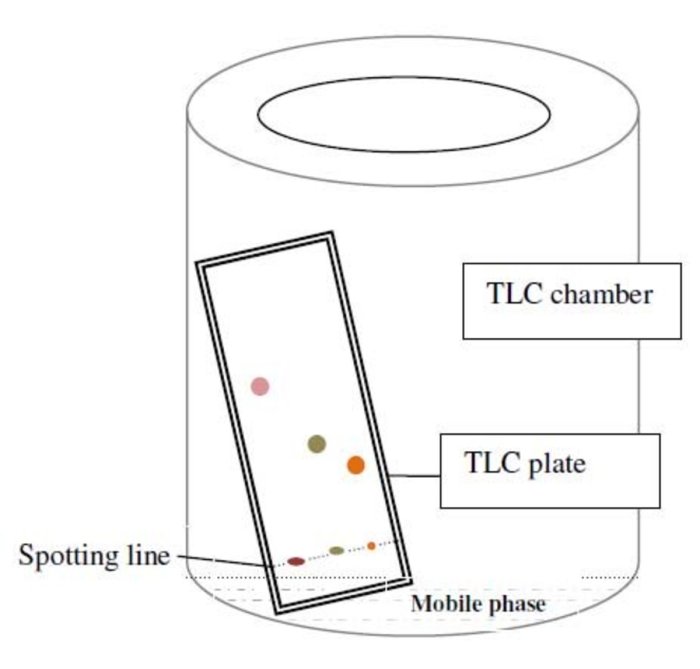
TLC is a chromatographic technique used to separate and identify compounds based on their different rates of migration on a stationary phase.
The TLC system consists of a stationary phase (a thin layer of adsorbent material, typically silica gel or alumina) coated on a glass or plastic plate, a mobile phase (a solvent or mixture of solvents), and a sample (a mixture of compounds to be separated).
TLC Techniques and Procedures
TLC involves the following steps:
- Spotting:The sample is applied as a small spot near the bottom of the TLC plate.
- Developing:The plate is placed in a developing chamber containing the mobile phase, which migrates up the plate by capillary action.
- Visualizing:The separated compounds are visualized using UV light, chemical staining, or other methods.
TLC Applications, Thin layer chromatography exam questions
TLC is widely used in various fields, including:
- Chemistry:Identifying and separating organic compounds, reaction monitoring, and purity analysis.
- Biology:Analyzing lipids, amino acids, and other biomolecules.
- Forensics:Identifying drugs, explosives, and other substances in forensic investigations.
TLC Optimization
Factors affecting TLC results include:
- Stationary phase:Type of adsorbent, particle size, and layer thickness.
- Mobile phase:Composition, polarity, and volume.
- Sample preparation:Concentration, volume, and purity.
Optimization involves adjusting these factors to improve separation, resolution, and detection sensitivity.
TLC Troubleshooting
Common TLC problems include:
- Streaking:Caused by uneven sample application or mobile phase flow.
- Tailing:Occurs when a compound interacts too strongly with the stationary phase.
- Ghosting:Faint spots that appear after visualization, indicating incomplete separation.
Solutions involve adjusting sample preparation, mobile phase composition, or development conditions.
TLC Data Analysis
TLC data is analyzed using:
- Rf values:Ratio of the distance traveled by the compound to the distance traveled by the solvent front.
- Peak areas:Measure of the amount of compound present, determined using densitometry.
Rf values and peak areas can be used to identify and quantify compounds.
FAQs
What is the purpose of thin layer chromatography?
TLC is used to separate and identify compounds in a mixture based on their different interactions with a stationary and mobile phase.
What are the key components of a TLC system?
A TLC system typically consists of a stationary phase (TLC plate), a mobile phase (solvent), and a sample to be analyzed.
How is a TLC experiment performed?
A TLC experiment involves spotting the sample onto the TLC plate, developing the plate in a solvent, and visualizing the separated compounds using appropriate techniques.
What factors can affect TLC results?
Factors such as the choice of stationary and mobile phases, sample preparation, and development conditions can influence TLC results.
How is TLC data analyzed?
TLC data is analyzed by calculating Rf values and peak areas, which provide information about the identity and quantity of the separated compounds.