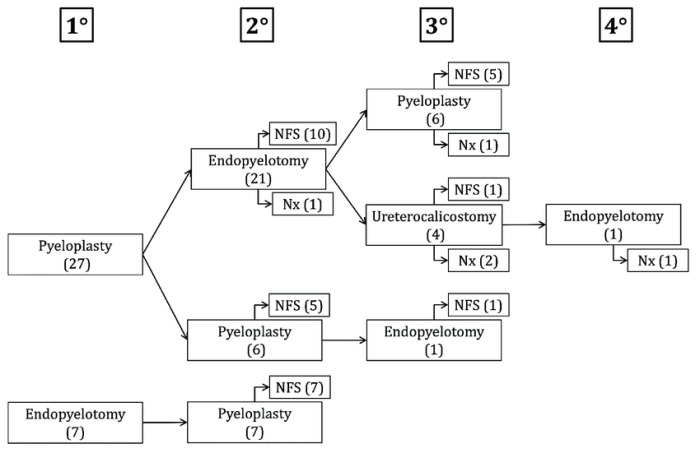
Rank the three carbocations shown in terms of increasing stability. Carbocations are positively charged carbon atoms that are formed when a carbon atom loses an electron. The stability of a carbocation is determined by the number of alkyl groups attached to the carbon atom.
The more alkyl groups attached, the more stable the carbocation. This is because the alkyl groups donate electrons to the carbocation, which helps to stabilize the positive charge.
The three carbocations shown are:
- CH3+
- (CH3)2CH+
- (CH3)3C+
The most stable carbocation is (CH3)3C+ because it has the most alkyl groups attached to the carbon atom. The least stable carbocation is CH3+ because it has the fewest alkyl groups attached to the carbon atom.
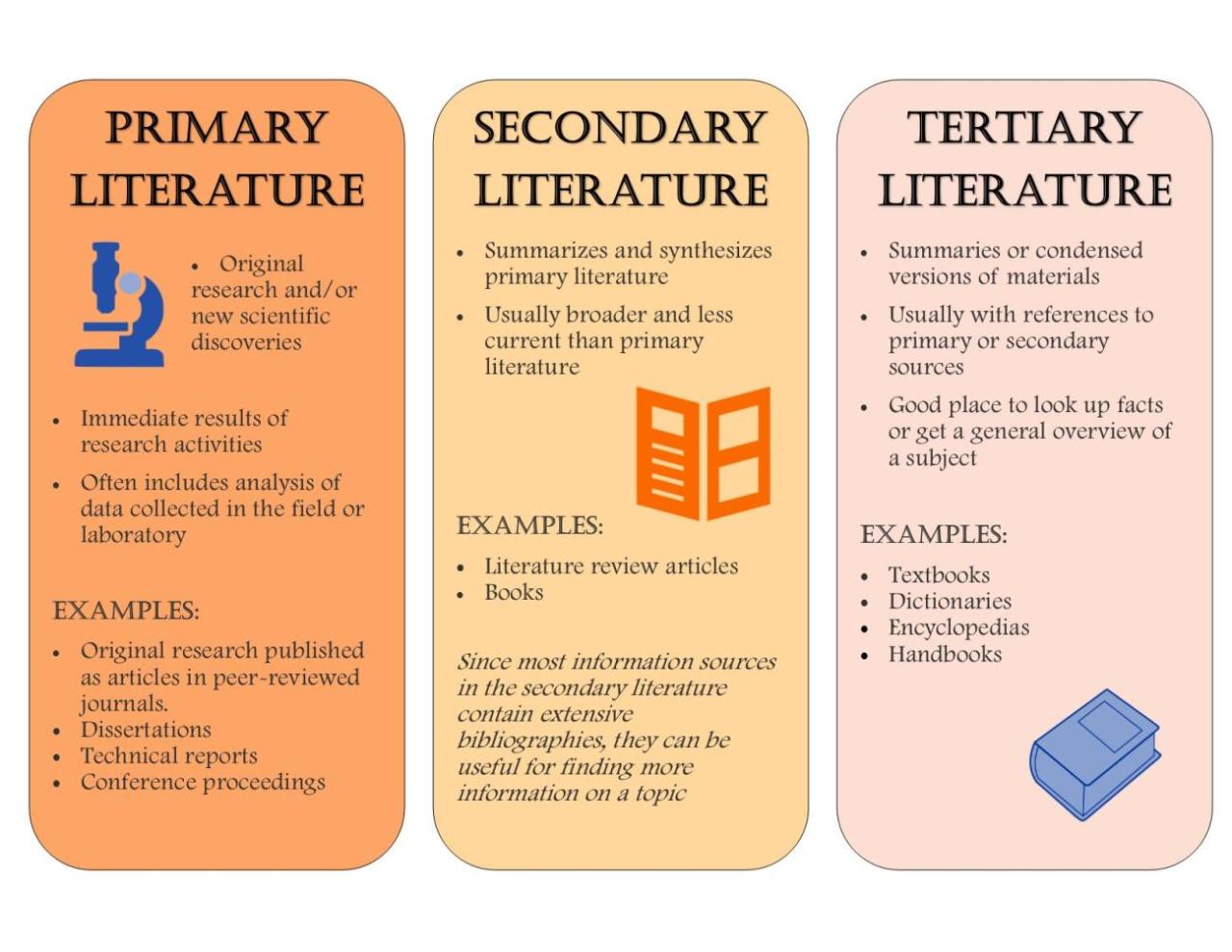
Carbocation Stability Basics
Carbocation stability is a measure of the relative reactivity of a carbocation. A more stable carbocation is less reactive and will be more likely to form and exist in a reaction. Carbocations are classified as primary, secondary, or tertiary, based on the number of carbon atoms bonded to the positively charged carbon atom.
Primary carbocations are the least stable, followed by secondary carbocations, and then tertiary carbocations. This is because the more alkyl groups that are bonded to the positively charged carbon atom, the more electron donating groups there are, which helps to stabilize the positive charge.
Resonance and Carbocation Stability
Resonance is a chemical phenomenon that occurs when a molecule or ion has two or more possible Lewis structures. In the case of carbocations, resonance can occur when the positively charged carbon atom is adjacent to a double bond. This allows the positive charge to be delocalized over the two carbon atoms of the double bond, which makes the carbocation more stable.
The more resonance structures that a carbocation has, the more stable it will be.
Hyperconjugation and Carbocation Stability
Hyperconjugation is a chemical phenomenon that occurs when a sigma bond is adjacent to a pi bond. In the case of carbocations, hyperconjugation can occur when the positively charged carbon atom is adjacent to a C-H bond. This allows the positive charge to be delocalized over the hydrogen atom of the C-H bond, which makes the carbocation more stable.
The more hyperconjugation structures that a carbocation has, the more stable it will be.
Inductive Effects and Carbocation Stability, Rank the three carbocations shown in terms of increasing stability
Inductive effects are the effects that substituents have on the electron density of a molecule or ion. In the case of carbocations, inductive effects can be either electron donating or electron withdrawing. Electron donating groups will make the carbocation more stable, while electron withdrawing groups will make the carbocation less stable.
The more electron donating groups that are bonded to the positively charged carbon atom, the more stable the carbocation will be.
Carbocation Rearrangements
Carbocation rearrangements are chemical reactions that occur when a carbocation rearranges to a more stable carbocation. Carbocation rearrangements can occur through a variety of mechanisms, including hydride shifts, alkyl shifts, and ring expansions. The most common type of carbocation rearrangement is the 1,2-hydride shift, which occurs when a hydrogen atom migrates from a carbon atom adjacent to the positively charged carbon atom to the positively charged carbon atom.
This results in the formation of a more stable carbocation.
General Inquiries: Rank The Three Carbocations Shown In Terms Of Increasing Stability
What is a carbocation?
A carbocation is a positively charged carbon atom.
What is the stability of a carbocation?
The stability of a carbocation is determined by the number of alkyl groups attached to the carbon atom.
What are the factors that affect carbocation stability?
The factors that affect carbocation stability include resonance, hyperconjugation, and inductive effects.